Note: This trial has now been completed.
Desert Institute for Spine Care has been selected to participate in the DASCOR™ disc nucleus replacement pilot clinical study.
This pilot clinical study is designed to evaluate the initial safety and effectiveness of the DASCOR™ Disc Arthroplasty device in subjects with single-level degenerative disc disease (DDD) of the lumbar spine from L2 to the sacrum and to provide data for use in hypothesis and endpoint determination for the pivotal study.
This study is a multicenter, prospective, pilot study in which 20 qualified subjects will be enrolled at up to five investigational sites. Study subjects will undergo surgery to implant the DASCOR™ Disc Arthroplasty device and will be followed postoperatively at predetermined intervals to evaluate safety and effectiveness.
The DASCOR™ Disc Arthroplasty device is an in-situ, curable polymer that is designed to replace the degenerated nucleus and restore the disc function in subjects with single-level DDD of the lumbar spine from L2 to the sacrum.
The DASCOR™ Disc Arthroplasty System replaces an abnormal disc nucleus with an artificial device that conforms to the disc nucleus space and is designed to perform like a natural healthy nucleus. The DASCOR™ device is different from an entire artificial disc replacement, where the entire spinal disc – not just the nucleus (the inner portion of the disc) – is replaced. Unlike total disc replacement, the DASCOR™ procedure is minimally invasive and preserves the anatomy of the spine.
The following images depict how the DASCOR™ Disc Arthroplasty System works:
STEP 1: The surgeon accesses the spine creating a small entry site into the annulus or outer portion of the disc.

STEP 2: The surgeon uses a series of instruments to perform a Total Nucleus Removal (TNR).
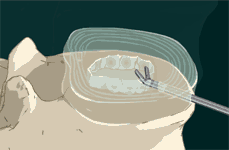
STEP 3: After total nucleus removal, a sizing/diagnostic balloon is inserted into the disc space.
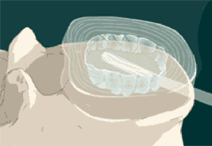
STEP 4: The balloon is filled with contrast solution that allows it to be seen on fluoroscopy. Then images of the balloon are taken to ensure proper positioning and sizing of the final implant. The contrast material and catheter are then removed.
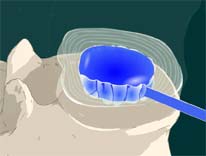
STEP 5: Another catheter with a balloon is inserted into the disc space.
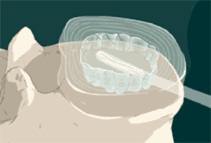
STEP 6: The mixed polymer passes through the catheter, as a liquid, filling the balloon and the disc space. The implant cures to form a firm but pliable implant that conforms to the individual’s anatomy. The polymer forms a single implant that remains in place.
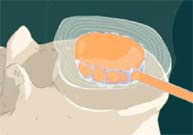
STEP 7: The catheter is then cut off at the edge of the implant and removed. The remaining implant is designed to restore the original disc function and now replaces the diseased nucleus.

To view an animation of the DASCOR™ Disc Arthroplasty System procedure, please visit www.discdyn.com and click on the image of the spine in the center of the page.
Study sponsor: Disc Dynamics, Inc.
Subjects must meet all of the following inclusion criteria and none of the exclusion criteria. A highlight of the criteria are listed below:
Sites that are participating in the DASCOR™ pilot study
Caution: Investigational Device, Limited by United States Law to Investigational Use.