- Bryan Cervical Disc
- M6-C *
- Mobi-C *
- PCM Cervical Disc System
- ProDisc-C *
- Secure-C
- Simplify Disc
In short and long-term clinical studies, the success rates for cervical artificial disc replacement surgery are superior to the anterior cervical discectomy and fusion surgery.
Cervical artificial disc replacement has undergone rigorous clinical studies, resulting in an effective and safe treatment option for cervical disc degeneration with myelopathy or radicular symptoms.
In short and long-term clinical studies, the patient satisfaction and clinical success rates for artificial cervical disc (CDR) replacement surgery are equal at one-level surgery and superior at two-level surgery to the anterior cervical discectomy and fusion surgery (ACDF). Greater than 95% of patients who underwent CDR and 88% of patients who underwent ACDF were “very satisfied” at seven years.
The potential patient benefits of artificial disc replacement versus spinal fusion surgery may include:
The key to success with cervical artificial disc replacement is proper patient selection. Not all patients are candidates for disc replacement. Patients with severe degeneration, spinal instability, and significant osteoporosis are contraindicated for CDR. They may be better selected for ACDF surgery.

“In our clinical experience, ideal candidate patients undergoing cervical ADR have a faster return to activity, greater satisfaction, and lower rates of need for additional surgery.”
– Board-Certified Orthopedic Spine Surgeon Dr. Nima Salari
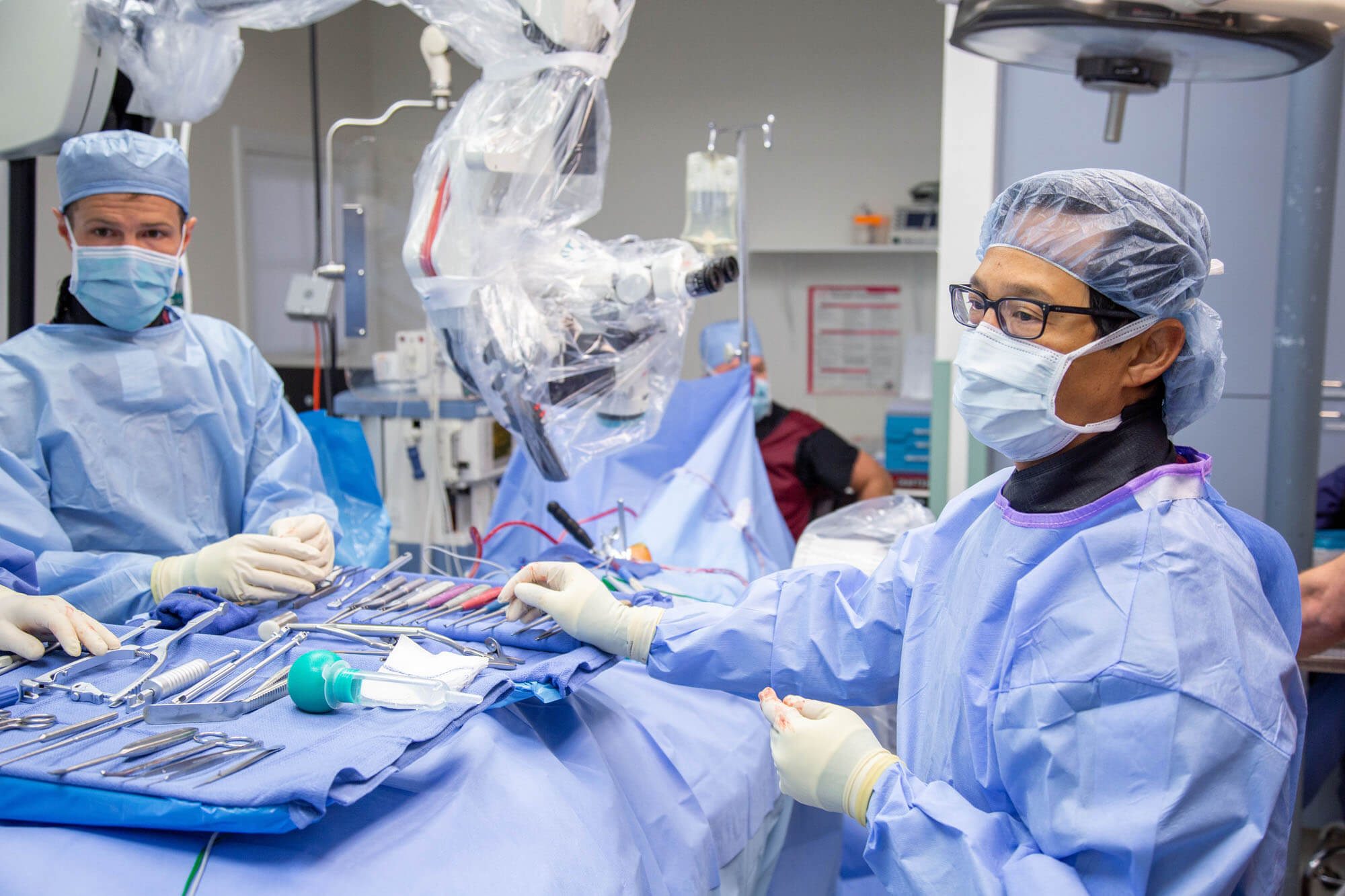
Artificial disc replacement (ADR) is a motion preservation spine surgery that removes a painful damaged spinal disc and replaces it with an artificial disc. This surgery is performed in the low back or the neck.
Cervical artificial disc replacement surgery is performed by a spine surgeon with the
patient under anesthesia.
While the patient is asleep lying on their back, the spine surgeon will make a one-to-two-inch
incision on the front of your neck. The surgeon then removes the affected cervical disc and replaces it with an artificial disc.
The surgery lasts about 1-2 hours as an outpatient minimally invasive procedure with patients going home a few hours after surgery.
While the patient is asleep lying on their back, a vascular surgeon assisting the spine surgeon will make a 2-inch incision in the abdomen. The muscle is gently retracted as are the deeper structures including the peritoneal sac and the major blood vessels are moved to the side to create access to the affected lumbar disc. The spine surgeon then removes the damaged degenerative disc and replaces it with an artificial disc. The patient is moved to recovery. The surgery lasts about 2-3 hours as an outpatient minimally invasive surgery. Most patients go home after a short recovery in the hospital.
Cervical artificial disc replacement surgery will restore your mobility to what it was before surgery. Removing and replacing the painful degenerative disc will relieve the spinal nerves’ pressure, eliminate pain, numbness, and tingling, and significantly improve your quality of life.
After surgery, you should have some pain and discomfort as with any surgery. Patients are discharged from the hospital in a few hours. Your surgeon will provide you with medication to control pain and specific guidelines for return to work and activities when leaving the hospital. Unlike spine fusion surgery, a cervical brace is not usually recommended. Most patients return to work and normal activities between two and six weeks.
As with any surgery, cervical spine surgery has potential risks and complications. There is always the risk for anesthesia complications, allergic reactions, and blood clotting with any spine surgery due to undiagnosed medical conditions such as those related to heart disease.
Potential risks and complications related to any cervical artificial disc replacement surgery may include:
|
|
Many of the major insurance carriers cover artificial disc replacement (ADR) based on risks, benefits, and cost of the procedure. However, if your insurance covers ADR, your specific health plan may have restrictions on which patients are approved.
Many insurers still consider cervical disc replacement surgery for degenerative disc disease investigational and experimental and therefore do not cover it. At DISC, our expert spine surgeons are significant advocates of cervical ADR. They often recommend this surgery for suitable candidates.
The limits vary from plan to plan but usually include the following to receive approval:
If you are a candidate, our insurance experts will assist you in getting the surgery approved.
Some health insurance carriers will deny a doctor’s recommendation for a lumbar or cervical artificial disc replacement. Each plan has restrictions on which patients are candidates for approval. When a patient has exhausted all internal and external appeals, they are left with two alternatives. Live with chronic back or neck pain or pay out-of-pocket for spine surgery. If you consider the out-of-pocket expenses, it is essential to understand all of the costs associated with the surgery before moving forward with your surgeon.
The total cost of disc replacement surgery can include the spine surgeon, the anesthesiologist, the vascular surgeon (lumbar surgery), the implant, and the facility’s fees. These fees can be bundled into one or two bills. Make sure to clarify the cost and billing with the surgeon’s office. The total cost of an artificial disc replacement surgery can range from $25,000 to $70,000, with cervical surgery on the lower end and lumbar on the upper end of the price range.
Other costs associated with artificial disc replacement will be pre-operative clearance, including evaluation, bloodwork, X-ray, MRI, and ECG studies. Post-operative, you may need physical therapy or rehabilitation. If paying out-of-pocket, this cost can be charged against your deductible.

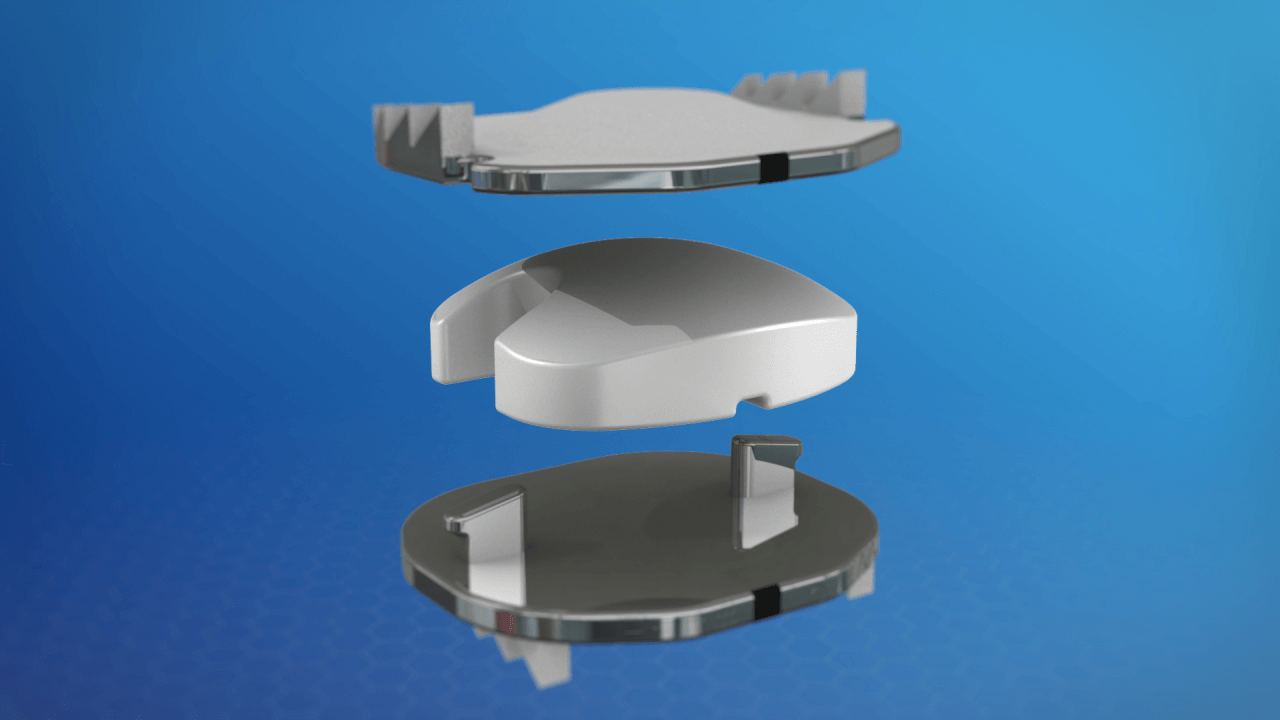
Over the past 30 years, there have been a variety of artificial disc replacement devices investigated as an alternative to spinal fusion. This surgical solution is also called spinal arthroplasty or total disc replacement.
The goal of the device is to maintain motion at the surgical level once the damaged disc has been removed and maintain normal biomechanics of the adjacent vertebrae levels above and below the new ADR.
The short answer is yes.
“An artificial disc replacement device may be replaced depending on the presenting symptoms and cause for consideration of the replacement. First, if the device has failed, but the segment is not too arthritic, you could consider a different device. Second, the failed device can be converted to a spinal fusion.” – Dr. Nima Salari
“Yes. Artificial discs can be implanted at multiple levels in the neck. The FDA has approved select disc replacements for two consecutive levels from C3-C7 in the cervical spine.
You may consider additional levels, but there are limited studies, and off-label use of the product is up to the discretion of the spine surgeon in charge of your care.” – Dr. Nima Salari
“Each of the approved artificial disc replacement devices is unique and has many different features. No one device is always the best fit for all patients. We encourage all patients to learn about each device, its makeup, and mechanisms which vary slightly.
Our goal here is not to promote one device over the other. The most common materials used are metal endplates with a polymer like a knee and hip replacement in the middle.” – Dr. Nima Salari
DISC offers a complete range of nonsurgical, ultra-minimally invasive, motion-preserving, and minimally invasive solutions for patients suffering from painful neck and back conditions.
Our spine health blog features up-to-date spine education and expert spine tips from our spine specialists here at DISC.
1635 East Myrtle Avenue Suite 100, Phoenix, AZ 85020, USA
18700 North 64th Drive Suite 105, Glendale, AZ 85308, USA
8630 East Vía de Ventura Suite 210, Scottsdale, AZ 85258, USA
3487 South Mercy Road, Gilbert, AZ 85297, USA
1635 East Myrtle Avenue Suite 400, Phoenix, AZ 85020, USA