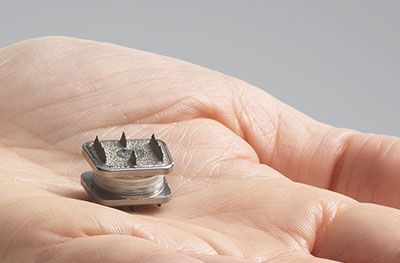
Phoenix, Arizona – May 15, 2019 – Desert Institute for Spine Care today announced the first implant in Arizona of a patient with the M6-C™ artificial cervical disc. Dr. Christopher A. Yeung performed the procedure on a 46 -year-old female patient suffering from cervical disc degeneration with a herniated disc. Recently approved by the U.S. Food and Drug Administration, the M6-C disc was designed as an innovative option for patients needing artificial disc replacement as an alternative to spinal fusion. Featuring a shock-absorbing nucleus and fiber annulus that work together to mimic the anatomic structure of a natural disc, the M6-C device is the only artificial cervical disc available in the U.S. that enables compression or “shock absorption” at the implanted level. The disc also provides a controlled range of motion when the spine transitions in its combined complex movements.
“The M6-C disc represents the continued evolution of artificial cervical disc technology,” said Yeung. “Compared to all the other artificial disc replacement options, the M6-C has the most natural and anatomical movement and we are excited to be able to offer this next-generation disc to patients suffering from cervical disc degeneration.”
The M6-C disc is designed to mimic the spine’s natural structure and movement, including backward and forward, side to side, up and down, and rotate left and right. By allowing the spine to move naturally, the M6-C artificial disc potentially minimizes stress to adjacent discs and other vertebral structures.
The M6-C disc received U.S. Food and Drug Administration (FDA) approval in February 2019. Approval was based on clinical data from a U.S. Investigational Device Exemption (IDE) study that evaluated the safety and effectiveness of the M6-C artificial cervical disc compared to anterior cervical discectomy and fusion (ACDF) for the treatment of symptomatic cervical radiculopathy with or without cord compression. Patients in the study who received the M6-C disc had a meaningful clinical improvement in pain scores including:
Prior to U.S. launch, the device had received CE Mark approval for distribution in the European Union and other international geographies. To date there have been close to 50,000 implants of the M6-C artificial cervical disc outside of the U.S.
Dr. Christopher Yeung served as the primary investigator in Arizona for the M6-C FDA study and with a 5 year history of implanting this disc replacement is the most experienced surgeon in the Southwest with this technology. DISC is the premier clinic in Arizona to offer the M6-C.
About Desert Institute for Spine Care: Desert Institute for Spine Care (DISC) is located in Arizona with offices in Phoenix, Scottsdale, Gilbert and soon Glendale. DISC is a comprehensive spine practice focused on endoscopic spine surgery and the latest minimally invasive treatment options to help preserve muscle and motion, and allow for quicker recovery times. DISC offers the full spectrum of spine care from non-surgical treatment to traditional open decompressive procedures, disc replacement, dynamic stabilization and fusion. All of the DISC spine surgeons are fellowship trained, use the latest innovative technologies and offer the broadest array of treatment options. Patients come from all over the world to receive their care. DISC is trusted by many professional teams, athletes as well as the weekend sports enthusiasts as they get back to their active lifestyles.
About Orthofix
The M6-C artificial cervical disc is manufactured and distributed by Orthofix, a global medical device company focused on musculoskeletal products and therapies. The Company’s mission is to improve patients’ lives by providing superior reconstruction and regenerative musculoskeletal solutions to physicians worldwide. Headquartered in Lewisville, Texas, Orthofix’s spine and orthopedic extremities products are distributed in more than 70 countries via the Company’s sales representatives and distributors. For more information about cervical disc degeneration and the M6-C disc, please visit the patient education website https://m6disc.com/.
Fact Sheet: What is the M6-C Artificial Cervical Disc?
Fact Sheet: What is Cervical Disc Degeneration?
Neck Pain Around the World Infographic