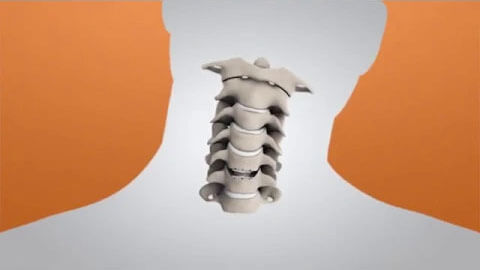
The M6-C disc was designed as an innovative option for patients needing artificial disc replacement as an alternative to spinal fusion. Featuring a shock-absorbing nucleus and fiber annulus that works together to mimic the anatomic structure of a natural disc, the M6-C device is the only artificial cervical disc available in the U.S. that enables compression or “shock absorption” at the implanted level. The disc also provides a controlled range of motion when the spine transitions in its combined complex movements.
The M6-C disc is designed to mimic the spine’s natural structure and movement, including backward and forward, side to side, up and down, and rotate left and right. By allowing the spine to move naturally, the M6-C artificial disc potentially minimizes stress to adjacent discs and other vertebral structures. If you would like to talk to one of our professionals about an MC-6 disc, contact us or make an appointment today!

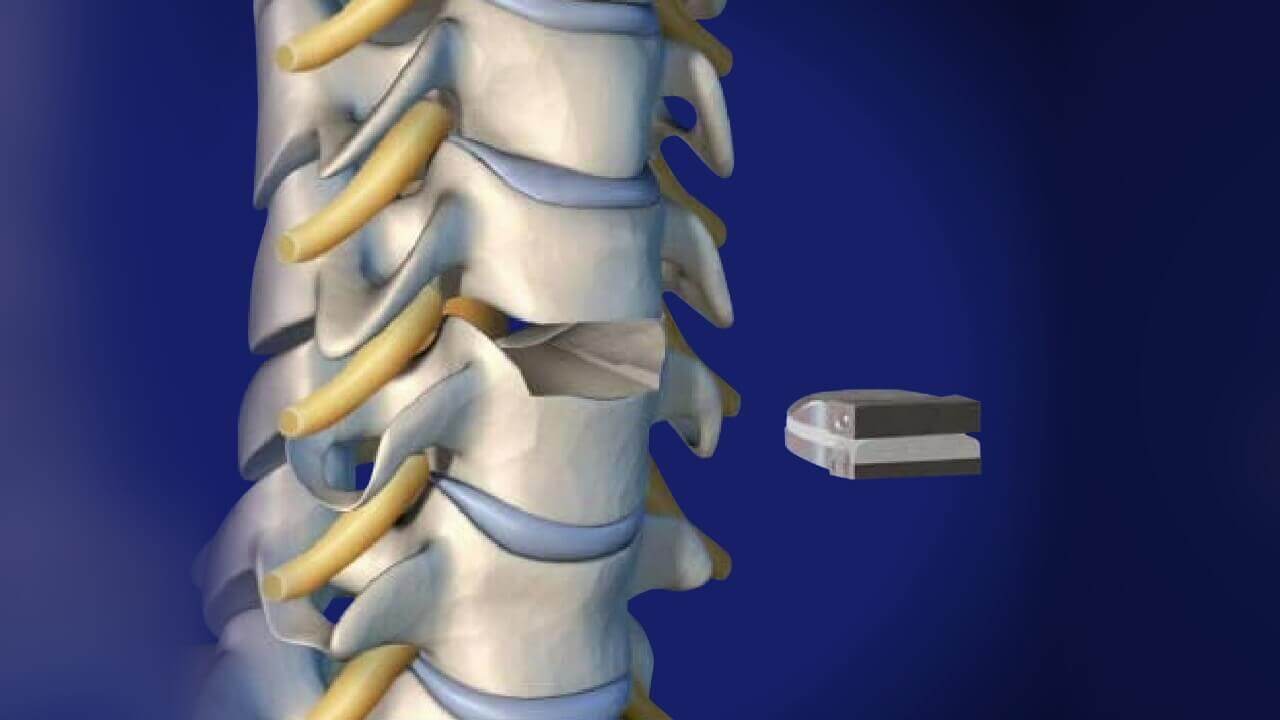
The M6-C™ artificial cervical disc is inserted through a small incision in the front of the neck after the patient’s native disc has been removed. The M6-C disc helps provide a controlled range of motion when the spine transitions in its combined complex movements.
The M6-C disc received U.S. Food and Drug Administration (FDA) approval in February 2019. Approval was based on clinical data from a U.S. Investigational Device Exemption (IDE) study that evaluated the safety and effectiveness of the M6-C artificial cervical disc compared to anterior cervical discectomy and fusion (ACDF) for the treatment of symptomatic cervical radiculopathy with or without cord compression.
The M6-C disc is the only disc available in the U.S. designed with a shock-absorbing nucleus and fiber annulus that works together to replicate the controlled range of movement and cushioning effect of the natural disc.
Patients in the study who received the M6-C disc had a meaningful clinical improvement in pain scores.
91.2 percent of patients who received the M6-C disc reported an improvement in neck pain compared to 77.9 percent in patients who underwent the ACDF procedure.
90.5 percent of the M6-C patients reported improvement in arm pain scores compared to 79.9 percent in ACDF patients.
Prior to the U.S. launch, the device had received CE Mark approval for distribution in the European Union and other international geographies. To date, there have been close to 50,000 implants of the M6-C artificial cervical disc outside of the U.S.
Dr. Christopher Yeung served as the primary investigator in Arizona for the M6-C FDA study and with a 5 year history of implanting this disc replacement is the most experienced surgeon in the Southwest with this technology. DISC is the premier clinic in Arizona to offer the M6-C.
The M6-C artificial cervical disc is manufactured and distributed by Orthofix, a global medical device company focused on musculoskeletal products and therapies. The Company’s mission is to improve patients’ lives by providing superior reconstruction and regenerative musculoskeletal solutions to physicians worldwide.
Headquartered in Lewisville, Texas, Orthofix’s spine and orthopedic extremities products are distributed in more than 70 countries via the Company’s sales representatives and distributors. For more information about cervical disc degeneration and the M6-C disc, please visit the patient education website.

When your neck pain prohibits you from working and living an active physical lifestyle, you should consult with an expert spine surgeon.
Our spine health blog features up-to-date spine education and expert spine tips from our spine specialists here at DISC.
1635 East Myrtle Avenue Suite 100, Phoenix, AZ 85020, USA
18700 North 64th Drive Suite 105, Glendale, AZ 85308, USA
8630 East Vía de Ventura Suite 210, Scottsdale, AZ 85258, USA
3487 South Mercy Road, Gilbert, AZ 85297, USA
1635 East Myrtle Avenue Suite 400, Phoenix, AZ 85020, USA